FAQs
Sample preparation
-
Consider purifying solvents used for chromatography or crystallisation such as petrol; nearly all commercial solvents contain involatile impurities which are insignificant when working with multi-gram quantities of compound, but become very objectionable when working on the small scale quantities high-field NMR has made possible.
- Precision 5 mm tubes must be used (available from CRL Stores). These should be periodically checked for cracks and for scratches around the bottom of the tubes, and discarded if necessary.
- NMR tubes that are shorter than 7 inches are already broken and should be discarded.
- Samples must be made up to a solvent depth of 4.0–4.5 cm. If you allow the liquid column to fall below 4 cm, the instrument will find it more difficult to lock and shim. Making samples too deep, although less detrimental, will dilute your sample and waste solvent.
- It is good practice to filter your samples before placing them in the NMR tube as floating debris or a cloudy solution often yield poor spectra.
- Ensure that sample tubes are clean on the outside before placing them in the instrument, to avoid contamination of the probe (which is a very serious matter!). If you break a tube whilst loading a turbine, clear up the mess with some methanol. Do not use chlorinated solvents as these will destroy the depth gauge plastic!
Open-access
You will typically require the following sample quantities for acceptable results:
- 200 MHz - ca. 5 mg
- 400 MHz - ca. 2 mg for 1H, or 20 mg for 13C
When handling oils, the quantity drawn up into a pipette by capillary action should be sufficient for a 1H spectrum.
Service
Service samples are most often run on the NEO600, which is equipped with a cryogenic probe and is thus substantially more sensitive than the open-access machines. This allows us to handle samples which are very dilute, going down to sub-milligram quantities.
Nevertheless, it is still in your best interests to submit as much sample as can be spared — this means that you are more likely to get your data back faster, and will also yield spectra of higher quality which are easier to interpret.
-
Check the sample qualityYou should first use one of the open-access 200 or 400 MHz spectrometers to collect a basic 1D 1H spectrum of your compound. This will allow you to check whether the structure corresponds to what you expected, as well as the integrity and quality of the sample.
-
Collect further data
Provided the sample is of sufficient quality, you may then need to collect further experiments on the open-access instruments to characterise the molecule, such as 1D 13C and 2D spectra. You should not collect all available experiments on a sample of unknown quality as these may waste instrument time; always assess the 1H spectrum first. -
Further experiments (if necessary)
If the data collected in this way is sufficient for your needs, you can stop here; otherwise, you may consider submitting samples to the NMR service or using the hands-on instruments. You are welcome to contact the NMR staff with any specific queries regarding collection or interpretation of data.
-
Make sure you choose an appropriate deuterated solvent — in particular, check their boiling and melting points to ensure that your solvent is still a liquid at the temperature you intend to study it at. Do not go within 10-15 °C of the boiling or melting points. Common solvents used for high-temperature work are toluene-d8 or DMSO-d6; and for low-temperature work, CD2Cl2, MeOD or DMSO-d6.
-
Use only NMR tubes made from Class A glass ("Pyrex"), such as the Norell S-400 ("Select Series") tubes. These can be obtained from Stores. Cheap / disposable tubes can warp or break at higher temperatures and will be rejected if submitted to the service.
-
Ensure your sample will be stable at the proposed temperature - the sample should be stable at the proposed temperature. If the sample does slowly decompose, it must not release any gaseous material that will increase the pressure within the NMR tube. This can break the NMR tube and cause damage to the NMR spectrometer.
A rolling baseline is commonly observed in 13C{1H} NMR spectra acquired on the NEO600. To avoid this, we use a 1D processing designed for cyroprobes. There is no issue when viewing the data in TopSpin, however in MestreNova, unless specific parameters are read in, the data cannot be phased. To correct this, complete the following steps in MestreNova:
Click File - Preferences - NMR - Import and ensure 'Apodization', 'Zero filling' and 'Linear Prediction' are checked in the 'Parameters' section:
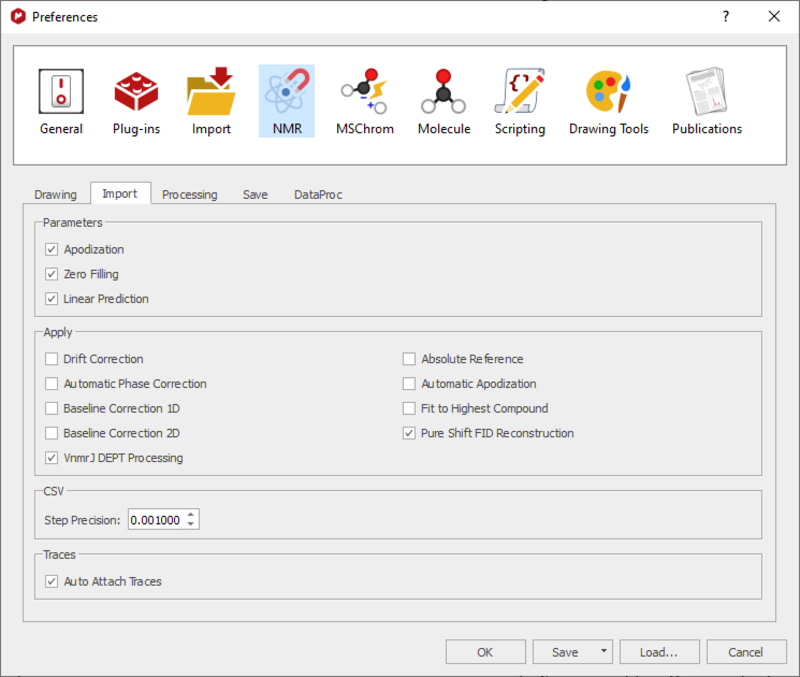
Re-open the software and the spectrum; it should look like a conventional 13C{1H} NMR spectrum. If this is not the case, please contact the NMR staff.


